
Advances in Neurometabolism and Nutrition: Projects
ANTIOXIDANT STATUS AND METHYLATION IN THE BRAIN
TOPIC: Vitamin B12 in human brain
PROJECT: Clinical evaluation of a brain-targeted nutraceutical formulation of vitamin B12 in autism and mild cognitive impairment (early Alzheimer’s disease)
To further explore the role of methylation in human brain we measured vitamin B12 levels in postmortem brain samples of subjects from fetal to 80 yrs of age. Total B12 levels decreased more than 10-fold across the lifespan, mainly after 50 yrs of age and mainly reflecting a decrease in the methylB12 species, which is needed for methylation activity. Such a decrease is not observed in blood measurements of B12, further indicating the brain-specific importance of methylation and epigenetic regulation. Autistic subjects and subjects with schizophrenia had brain B12 levels that were only one-third of control subjects of the same age. This work suggests that augmenting brain B12 levels might have therapeutic benefit. Consistent with this finding, clinical studies from a number of other investigators have reported encouraging effects of vitamin B12, folic acid and vitamin B6 supplementation in decreasing the rate of progression of early Alzheimer’s disease. Accordingly, we have undertaken development of a novel multi-component nutraceutical formulation to provide improved effectiveness in preventing Alzheimer’s disease and/or slowing its progression.
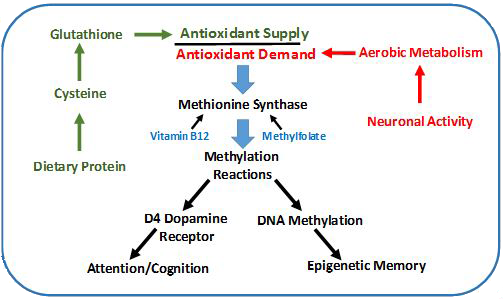
TOPIC: Oxidative metabolism and brain disorders
PROJECT: Analysis of antioxidant and methylation metabolite status in different brain regions of subjects with autism, schizophrenia, major depression and Alzheimer’s disease.
All cells depend upon energy-producing metabolic pathways, including aerobic and non-aerobic sources, and they must have adequate antioxidant capacity to avoid damage, especially from aerobic metabolism by mitochondria. While the human brain develops and functions within a unique metabolic environment that is optimized for its cognitive and memory functions, this unique metabolic environment also brings risk. Most important among these factors is the extraordinarily high level of aerobic metabolism in the brain, which consumes oxygen at a 10-fold higher rate than other tissues. At the same time, however, the available level of antioxidant resources in the brain is the lowest of any tissue. This precarious metabolic balance assures that the level of neural firing activity will strongly impact antioxidant levels, setting into motion changes in gene expression which constitute a form of learning and memory. During a person’s early years these activity-dependent changes in gene expression serve to incorporate experience into the developing neural networks, but if antioxidant resources fall short there can be neurodevelopmental consequences. Similarly, the occurrence of oxidative stress later in life can contribute to functional brain disorders such as schizophrenia and depression, while in older age it can lead to neurodegenerative disorders such as Alzheimer’s disease. Our studies of postmortem human brain were the first to demonstrate dynamic age-dependent changes in the vitamin B12 and folate-dependent enzyme (methionine synthase) across the lifespan. Recognition of the crucial importance of oxidative stress in brain disorders across the lifespan provides a guide for basic and clinical research while also opening the way to novel metabolism-based treatment strategies.
TOPIC: Epigenetic regulation
PROJECT: Evaluation of gene-specific DNA methylation patterns for antioxidant and methylation pathway enzymes in subjects with autism, schizophrenia, major depression and Alzheimer’s disease.
Over the past 15 years there has been an explosion of knowledge about how gene expression is regulated, revealing the central role of epigenetic mechanisms involving methylation of DNA and histone proteins. Since methylation reactions are very sensitive to antioxidant status, the energy demand associated with high rates of neuronal firing leads to changes in DNA and histone methylation and gene expression which can last for a lifetime, or even be transmitted across generations. The metabolic pathways which support methylation and antioxidant production are therefore essential for normal learning and memory capabilities, which involve epigenetic mechanisms. Moreover, our recent research has provided evidence that antioxidant-dependent epigenetic regulation appears to be fundamental for the overall program of development, including neural stem cell differentiation.
TOPIC: Environmental exposures affect the brain
PROJECT: Evaluate the dose-dependent impact of selected environmental toxins on antioxidant and methylation pathway metabolites in brain regions of laboratory animals.
There is increasing concern about the health impact of toxic chemical exposures, for example those arising from the environment, food ingredients, dental amalgams, vaccines, prescribed and illicit drugs etc. Almost all toxic exposures deplete antioxidant levels, which is in fact their mechanism of toxicity, and it is not surprising, therefore, that neurodevelopment and ongoing brain function are highly sensitive to toxic exposures. We have previously characterized the potent inhibitory effects of toxins such as lead, mercury, aluminum and arsenic on both antioxidant status and methylation activity. We further demonstrated that alcohol and morphine impair the production of glutathione (GSH) the principal antioxidant in all cells, as well as interfering with methylation and epigenetic regulation. Recognition of these relationships leads to metabolic treatment strategies capable of preventing and reversing the impact of environmental exposures.
TOPIC: Vitamin B12 in human brain
PROJECT: Clinical evaluation of a brain-targeted nutraceutical formulation of vitamin B12 in autism and mild cognitive impairment (early Alzheimer’s disease)
To further explore the role of methylation in human brain we measured vitamin B12 levels in postmortem brain samples of subjects from fetal to 80 yrs of age. Total B12 levels decreased more than 10-fold across the lifespan, mainly after 50 yrs of age and mainly reflecting a decrease in the methylB12 species, which is needed for methylation activity. Such a decrease is not observed in blood measurements of B12, further indicating the brain-specific importance of methylation and epigenetic regulation. Autistic subjects and subjects with schizophrenia had brain B12 levels that were only one-third of control subjects of the same age. This work suggests that augmenting brain B12 levels might have therapeutic benefit in autism and schizophrenia, consistent with several clinical studies from other investigators.
TOPIC: Gluten and casein intolerance
PROJECT: Influence of dietary casein and gluten on brain antioxidant levels across the lifespan.
An increasing number of people appear to be intolerant of gluten and casein-containing foods such as wheat and milk products, and a gluten-free/casein-free (GF/CF) diet has been reported to be beneficial in autism, schizophrenia, chronic fatigue syndrome and other disorders. In 2014 we published studies showing that opiate peptides released from both gluten and casein restrict transport of the amino acid cysteine, limiting antioxidant availability. Thus a GF/CF diet can help to increase antioxidant levels, which is especially important for individuals with low levels. Notably, the opiate peptide is not released from cow milk containing only the a2-type casein, resulting in higher antioxidant levels. These finding also has important implications for early development when breast milk or infant formula are the primary source of nutrition, illustrating that efficient absorption of macro- and micronutrients, in concert with a healthy microbiome, is essential for postnatal brain development.
TOPIC: Epigenetics and addiction
PROJECT: Evaluation of redox and gene-specific epigenetic changes in addiction-related brain regions after acute and chronic administration of morphine
Addiction is associated with epigenetic-related changes in gene expression and a number of drugs with addictive potential have effects on neuronal antioxidant metabolic pathways (e.g. opiates, alcohol, amphetamines, caffeine and nicotine). Consequently, we proposed that addiction reflects a state of epigenetic adaptation to the sustained presence of drugs which potently interfere with the relationship between antioxidant status and methylation. In other words, addictive drugs hijack the molecular mechanisms that provide for attention, learning and memory, allowing drug exposures to induce neurochemical changes in gene expression that substitute for normal experience. The current “opiate epidemic” emphasizes the importance of exploring the molecular mechanisms of addiction for the purpose of identifying more effective treatments.
TOPIC: Nutritional interventions
PROJECT: Clinical assessment of nutraceutical and nutrition-based interventions to augment brain redox and methylation status.
Recognition of the critical role of antioxidant and methylation status in autism and other brain disorders opens the way to treating and/or preventing these conditions with metabolic interventions, including nutritional supplements. Building upon insights gained from our previous studies, we have initiated development of novel nutraceuticals specifically designed to augment brain levels of vitamin B12. In addition, we have established commercial collaborations to evaluate the efficacy of nutrition-based products to improve redox and methylation status in the brain.
- Karhu E, Zukerman R, Eshraghi R, Mittal J, Deth R, Trivedi M, Castejon Ana, Mittal R, Eshraghi A. Nutritional Interventions for Autism Spectrum Disorder. Nutr Rev. 2019 Dec 26. pii: nuz09
- Hodgson NW, Waly MI, Trivedi MS, Power-Charnitsky V-A, Deth RC. Methylation-related metabolic effects of D4 dopamine receptor expression and activation. Transl Psychiatry. 2019 Nov 12;9(1):295.
- Ye Q, Trivedi M, Zhang Y, Böhlke M, Alsulimani H, Chang J, Maher T, Deth R, Kim J. Brain iron loading impairs DNA methylation and alters GABAergic function in mice. FASEB J. 2019 Feb;33(2):2460-2471.
- Padmanabhan S, Waly MI, Taranikanti V, Guizani1 N, Ali A, Rahman MS, Al-Attabi1 Z, Al-Malki1 RN, Al-Maskari SNM, Al-Ruqaishi BRS, Dong J, Deth RC. Folate/Vitamin B12 Supplementation Combats Oxidative Stress-associated Carcinogenesis in a Rat Model of Colon Cancer. Nutr Cancer. 2018 Oct 29:1-11.
- Eshraghi RS, Deth RC, Mittal R, Aranke M, Kay SS, Moshiree B, Eshraghi AA. Early Disruption of the Microbiome Leading to Decreased Antioxidant Capacity and Epigenetic Changes: Implications for the Rise in Autism. Front Cell Neurosci. 2018 Aug 15;12:256. doi:10.3389/fncel.2018.00256. eCollection 2018.
- Terhune TD, Deth RC. Aluminum Adjuvant-Containing Vaccines in the Context of the Hygiene Hypothesis: A Risk Factor for Eosinophilia and Allergy in a Genetically Susceptible Subpopulation? Int J Environ Res Public Health. 2018 May 3;15(5). pii: E901. doi:10.3390/ijerph15050901.
- Kern JK, Geier DA, Deth RC, Sykes LK, Hooker BS, Love JM, Bjørklund G, Chaigneau CG, Haley BE, Geier MR. Systematic Assessment of Research on Autism Spectrum Disorder (ASD) and Mercury Reveals Conflicts of Interest and the Need for Transparency in Autism Research. Sci Eng Ethics. 2017 Dec;23(6):1691-1718. doi: 10.1007/s11948- 017-9983-2.
- Schrier MS, Trivedi MS, Deth RC. Redox-Related Epigenetic Mechanisms in Glioblastoma: Nuclear Factor (Erythroid-Derived 2)-Like 2, Cobalamin, and Dopamine Receptor Subtype 4. Front Oncol. 2017 Mar 30;7:46.
- Deth R, Clarke A, Ni J, Trivedi M. Clinical evaluation of glutathione concentrations after consumption of milk containing different subtypes of β-casein: results from a randomized, cross-over clinical trial. Nutr J. 2016 Sep 29;15(1):82.
- Zang T, Pottenplackel LP, Handy DE, Loscalzo J, Dai S, Deth RC, Zhou ZS and Ma J. Comparison of Protein N-Homocysteinylation in Rat Plasma under Elevated HomocysteineUsing a Specific Chemical Labeling Method. Molecules 2016, 21, 1195; doi:10.3390/molecules21091195.
- Trivedi MS, Hodgson NW, Walker SJ, Trooskens G, Nair V, Deth RC. Epigenetic effects of casein-derived opioid peptides in SH-SY5Y human neuroblastoma cells. Nutr Metab (Lond). 2015 Dec 9;12:54. doi: 10.1186/s12986-015-0050-1. eCollection 2015. PMID: 26664459.
- Waly M, Power-Charnitsky V-A, Hodgson N, Sharma A, Audhya T, Zhang Y and Deth R. Alternatively Spliced Methionine Synthase in SH-SY5YNeuroblastoma Cells: Cobalamin and GSH Dependence and Inhibitory Effects of NeurotoxicMetals and Thimerosal. Oxidative Medicine and Cellular Longevity 2016:6143753. doi: 10.1155/2016/6143753.
- Zhang Y, Hodgson NW, Trivedi MS, Abdolmaleky HM, Fourniere M, Cuenod M, Quang Do K, Deth RC. Decreased brain levels of vitamin B12 in aging, autism and schizophrenia. PLOS ONE 2016 Jan 22;11(1):e0146797. doi: 10.1371/journal.pone.0146797. eCollection PMID: 26799654.
- Zhang Y, Hodgson N, Trivedi M and Deth RC. Neuregulin-1 promotes glutathione-dependent neuronal cobalamin metabolism by stimulating cysteine uptake. Oxidative Medicine and Cellular Longevity. 2016;2016:3849087.
- Trivedi M, Zhang Y, Lopez-Toledano M, Clarke A, Deth R. Differential neurogenic effects of casein-derived opioid peptides on neuronal stem cells: implications for redox-based epigenetic changes. J Nutr Biochem. 2016 Nov;37:39-46.
- Trivedi MS, Deth RC. Redox-based Epigenetic status in Drug Addiction: Potential mediator of drug-induced gene priming phenomenon and use of metabolic intervention for symptomatic treatment in drug addiction. Frontiers in Neuroscience 2015; 8:444.
- Terhune TD, Deth RC. A role for impaired regulatory T cell function in adverse responses to aluminum adjuvant-containing vaccines in genetically susceptible individuals. Vaccine. 2014, 32(40):5149-55.
- Walach, H, Mutter, J and Deth, RC. Inorganic Mercury and Alzheimer’s Disease-Results of a Review and a Molecular Mechanism. pp 593-602 in Diet and Nutrition in Dementia and Cognitive Decline ed. C. R. Martin and V.R. Preedy, 2015, Elsevier, (London, UK).
- Trivedi, MS, Shah, JS, Al-Mughairy, S, Hodgson NW, Simms B, Trooskens BA, Van Criekinge W, Deth RC. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J Nutritional Biochemistry 2014 ;25(10):1011-8.
- Trivedi M, Shah J, Hodgson N, Byun HM, Deth R. Morphine induces redox-based changes in global DNA methylation and retrotransposon transcription by inhibition of excitatory amino Acid transporter type 3-mediated cysteine uptake. Mol Pharmacol. 2014; 85(5):747-57.
- Hodgson, N.W, Waly, M.I., Al-Farsi, Y.M., Al-Sharbati, M.M., Al-Farsi, O., Ali, A., Ouhtit, A., Zang, T., Zhou,, Z.S., Deth, R.C. Decreased glutathione and elevated hair mercury levels are associated with nutritional deficiency-based autism in Oman. Exp. Biol. Med. (Maywood). 2013, 239(6):697-706.
- Deth R, Trivedi MS, Hodgson NW, Muratore CR and Waly MI. Redox-Methylation Theory and Autism. In: Comprehensive Guide to Autism, Springer, 2013.
- Deth RC. Autism: a redox/methylation disorder. Glob Adv Health Med. 2013; 2(6):68-73.
- Yeter D, Deth R, Kuo H-C. Mercury Promotes Catecholamines Which Potentiate Mercurial Autoimmunity and Vasodilation: Implications for Inositol 1,4,5-Triphosphate 3-Kinase C Susceptibility in Kawasaki Syndrome. Korean Circ J. 2013; 43:581-591.
- Kocsis B, Lee P, Deth R. Enhancement of gamma activity after selective activation of dopamine D4 receptors in freely moving rats and in a neurodevelopmental model of schizophrenia. Brain Struct Funct. 2014 Nov; 219(6):2173-80. doi: 10.1007/s00429-013-0607-6.
- Hodgson N, Trivedi M, Muratore C, Li S, Deth R. Soluble Oligomers of Amyloid-β Cause Changes in Redox State, DNA Methylation, and Gene Transcription by Inhibiting EAAT3 Mediated Cysteine Uptake. J Alzheimers Dis. 2013; 36(1):197-209.
- Muratore CR, Hodgson NW, Trivedi MS, Abdolmaleky HM, Persico AM, Lintas C, De la Monte S, Deth RC. Age-dependent decrease and alternative splicing of methionine synthase mRNA in human cerebral cortex and an accelerated decrease in autism. PLoS One. 2013;8(2):e56927.
- Al-Farsi YM, Waly MI, Deth RC, Al-Sharbati MM, Al-Shafaee M, Al-Farsi O, Al-Khaduri MM, Al-Adawi S, Hodgson NW, Gupta I, Ouhtit A. Impact of nutrition on serum levels of docosahexaenoic acid among Omani children with autism. Nutrition. 2013 (13) 00196-2.
- Al-Farsi YM, Waly MI, Al-Sharbati MM, Al-Shafaee MA, Al-Farsi OA, Al-Khaduri MM, Gupta I, Ouhtit A, Al-Adawi S, Al-Said MF, Deth RC. Levels of Heavy Metals and Essential Minerals in Hair Samples of Children with Autism in Oman: a Case-Control Study. Biol Trace Elem Res. 2013 Feb;151(2):181-6.
- Raymond, L.J., Deth, R.C., Ralston, N.V.C. Potential Role of Selenoenzymes and Antioxidant Metabolism in relation to Autism Etiology and Pathology. Autism Res Treat. 2014:164938.
- Kern, J.K., Geier, D.A., Geier, M.R., Deth, R.C. Are ASD and ADHD a Continuum? A Comparison of Pathophysiological Similarities Between the Disorders. Journal of Attention Disorders 2015 Sep;19(9):805-27. doi: 10.1177/1087054712459886.
- Trivedi, M.S., Deth, R.C. Role of a Redox-Based Methylation Switch in mRNA Life Cycle (Pre-and Post-Transcriptional Maturation) and Protein Turnover: Implications in Neurological Disorders. Frontiers in Neuroscience. 2012;6:92.
- Deth, R.C. Genomics, intellectual disability, and autism. (Letter to the Editor) N Engl J Med. 2012; 366:2231-2.
- Al-Farsi, Y.M., Al-Sharbati, M.M., Waly, M.I., Al-Farsi, O.A., Al-Shafaee, M.A., Al-Khaduri, M.M., Trivedi, M.S., Deth, R.C.. Effect of suboptimal breast-feeding on occurrence of autism: A case-control study. Nutrition. 2012 Jul;28(7-8):e27-32.
- Waly, M.I., Hornig, M., Trivedi, M., Hodgson, N., Kini, R., Ohta, A., Deth, R. Prenatal and Postnatal Epigenetic Programming: Implications for GI, Immune, and Neuronal Function in Autism. Autism Research and Treatment. 2012 doi:10.1155/2012/190930.
- Dufault, R., Lukiw, W.J., Crider, R., Schnoll, R., Wallinga, D., Deth, R. A macroepigenetic approach to identify factors responsible for the autism epidemic in the United States. Clinical Epigenetics. 2012 Apr 10;4(1):6.
- Terhune, T.D., Deth, R.C. How Aluminum Adjuvants Could Promote and Enhance Non-target IgE Synthesis in a Genetically-vulnerable Sub-population. J Immunotoxicology. 2013 Apr-Jun;10(2):210-22.
- Yeter, D., Deth, R. ITPKC susceptibility in Kawasaki syndrome as a sensitizing factor for autoimmunity and coronary arterial wall relaxation induced by thimerosal's effects on calcium signaling via IP3. Autoimmunity Reviews. 2012 Oct;11(12):903-8. doi:10.1016/j.autrev.2012.03.006.
- Ali, A., Waly, M.I., Al-Farsi, Y.M., Essa, M.M, Al-Sharbati, M.M., and Deth, R.C. Hyperhomocysteinemia among Omani autistic children:a case-control study. Acta Biochimica Polonica 58: 547-51, 2011.
- Deth, R.C., Hodgson, N.W., Trivedi, M.S., Muratore, C.R., and Waly, M.I. Autism: a neuroepigenetic disorder. Autism Science Digest 3: 7-18 (2012).
- Al-Farsi, Y.M., Al-Sharbati, M.M., Waly, M.I., Al-Farsi, O.A, Al-Shafaee, M.A. and Deth, R.C. Malnutrition among preschool-aged autistic children in Oman. Research in Autism Spectrum Disorders. 5 1549-52 (2011).
- Deth, R.C. The Redox/Methylation Hypothesis of Autism. U.S. Psychiatry 3: 48-52 (2010).
- Waly, M.I., Kharbanda, K.K. and Deth, R.C. Ethanol Lowers Glutathione in Rat Liver and Brain and Inhibits Methionine Synthase in a Cobalamin-dependent Manner. Alcoholism: Clinical and Experimental Research 35: 277-83 (2011).
- Deth, R.C., Waly, M., Muratore, C. and Hodgson, N. Redox Imbalance and the Metabolic Pathology of Autism. In Developmental Neurotoxicology Research: Principles, Models, Techniques, Strategies and Mechanisms Section V: Autism Spectrum Disorders Section Ed. Isaac Pessah, John Wiley and Sons, Hoboken, (2010).
- Mutter, J., Curth, A., Naumann, J., Deth, R. and Walach, H. Does Inorganic Mercury Play a Role in Alzheimer's Disease? A Systematic Review and an Integrated Molecular Mechanism. J Alzheimer’s Disease 22: 357-374 (2010).
- Deth, R.C. The Redox/Methylation Hypothesis of Autism: A Molecular Mechanism for Heavy Metal-induced Neurotoxicity. In Autism: Oxidative Stress, Inflammation and ImmuneAbnormalities, A. Chauhan, V. Chauhan, W.T. Brown Eds., Taylor & Francis/CRC Press, Boca Raton (2009).
- Kane, P., Cartaxo, A., and Deth, R.C. Nutritional issues in the causation and treatment of autism. In Food and Nutrients in Disease Management, Ingrid Kohlstadt Ed., CRC Press, Boca Raton (2008).
- Deth, R.C., Muratore, C., and Waly, M. Oxidative stress in autism and its implications for dopamine-stimulated phospholipid methylation in Neurochemical Basis of Autism: Molecules to Minicolumns, Gene Blatt Ed., Springer, New York (2009).
- Deth, R., Muratore, C., Benzecry, J., Power-Charnitsky, V., and Waly, M. How environmental and genetic factors combine to cause autism: A Redox/Methylation Hypothesis. Neurotoxicology 29: 190-201 (2007).
- Kuznetsova, A.Y., and Deth, R.C. A model for gamma oscillations induced by D4 dopamine receptor-mediated phospholipid methylation. J. Computational Neuroscience 24 (3):314-29 (2008).
- Culley, D.J., Raghavan, S.V., Waly, M., Baxter, M.G., Yukhananov, R., Deth, R.C. and Crosby, G. Nitrous oxide decreases cortical methionine synthase transiently but produces lasting memory impairment in aged rats. Anesthesia and Analgesia 105: 83-88 (2007).
- Deth, R.C., Kuznetsova, A. and Waly, M. Attention-related signaling activities of the D4 dopamine receptor in Cognitive Neuroscience of Attention, Michael Posner Ed., Guilford Publications Inc., New York (2004). pp 269-282.
- Waly, M., Banerjee, R., Choi, S.W., Mason, J., Benzecry, J., Power-Charnitsky, V.A, Deth, R.C. PI3-kinase regulates methionine synthase: Activation by IGF-1 or dopamine and inhibition by heavy metals and thimerosal. Molecular Psychiatry 9: 358-370 (2004).
- Deth, R.C., Sharma, A. and Waly, M. Dopamine-stimulated solid-state signaling: A novel role for single-carbon folates in human attention. Proc. 12th Int. Symp. Chem. Pteridines and Folates. Kluwer Academic Press (2002).
- Sharma, A. and Deth, R.C. Protein kinase C regulates basal and D4 dopamine receptor-mediated phospholipid methylation in neuroblastoma cells. Eur. J. Pharmacol. 427: 83-90 (2001).
- Zhao, R., Chen, Y., Tan, W., Waly, M., Malewicz, B., Stover, P., Rosowsky, A. and Deth, R.C. Influence of single-carbon folate and de novo purine synthesis pathways on D4 dopamine receptor-mediated phospholipid methylation. J. Neurochem. 78: 788-796 (2001).
- Sharma, A., Kramer, M., Wick, P.F., Liu, D., Chari, S., Shim, S., Tan, W.-B., Ouellette, D., Nagata, M., DuRand, C., Kotb, M. and Deth, R.C. Dopamine D4 receptor-mediated methylation of membrane phospholipids and its implications for mental illnesses such as schizophrenia. Molecular Psychiatry 4: 235-246 (1999).